Pyrolysis: Core Characteristics and Key Differences from Combustion & Gasification
This is a subtitle for your new post
When it comes to managing organic waste, biomass, or even plastic materials, thermal conversion technologies are among the most effective solutions. But with terms like pyrolysis, combustion, and gasification thrown around interchangeably, it’s easy to mix them up—even though they’re fundamentally different processes with distinct outcomes. Today, we’re breaking down pyrolysis: its core characteristics, and how it stands apart from combustion and gasification, so you can understand why it’s gaining traction as a sustainable option for waste treatment and energy recovery.
First, Let’s Define the Basics: What Is Pyrolysis?
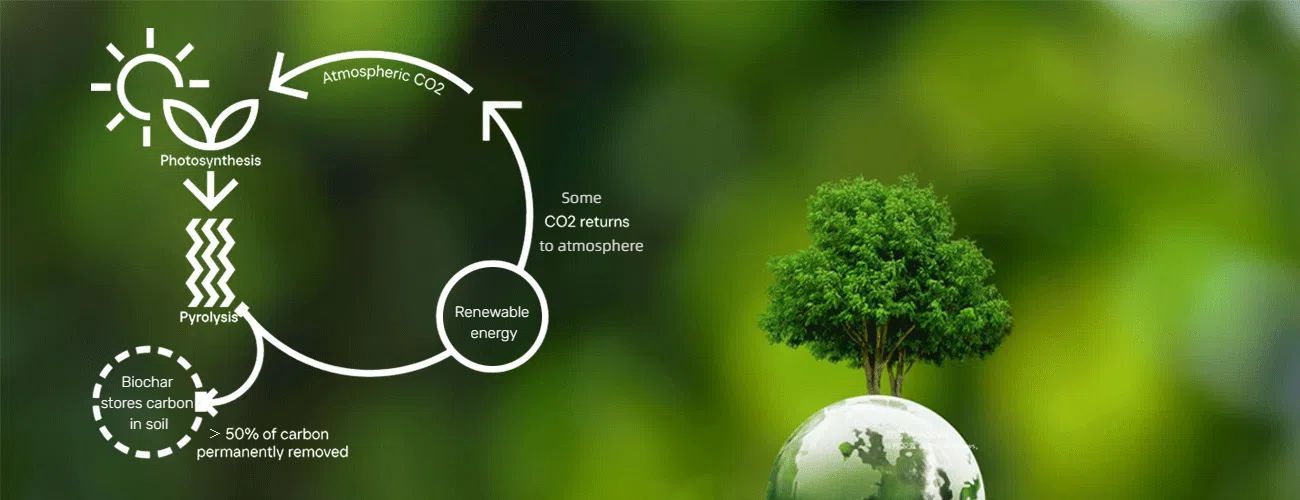
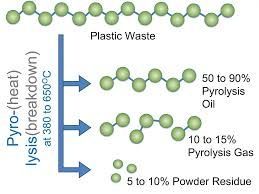
Pyrolysis is a thermal decomposition process that breaks down organic materials (known as “feedstock”) in the absence of oxygen (or in an oxygen-lean environment). Unlike processes that rely on oxidation, pyrolysis uses high temperatures—typically between 400°C and 800°C, though some advanced systems can go higher—to break chemical bonds in the feedstock, converting it into three main products: solid (char), liquid (bio-oil or pyrolysis oil), and gas (syngas, a mixture of hydrogen, methane, and carbon monoxide). More in pyrolysis unit.
The key here is “no oxygen.” Without oxygen, there’s no combustion (burning) of the feedstock—only thermal cracking. This absence of oxidation is what gives pyrolysis its unique advantages, including cleaner products and greater control over the end results.
Core Characteristics of Pyrolysis
To truly grasp pyrolysis, let’s highlight its most defining features—traits that set it apart from other thermal conversion methods:
1. Oxygen-Free (Anaerobic) Environment
This is the non-negotiable core of pyrolysis. By excluding oxygen, the process avoids oxidation reactions that would otherwise produce large amounts of carbon dioxide (CO₂), nitrogen oxides (NOₓ), and other harmful emissions. Instead, the feedstock is broken down into stable, energy-dense products that can be reused or refined.
2. Three Product Streams (Solid, Liquid, Gas)
Unlike combustion (which primarily produces heat and ash) or gasification (which focuses on syngas production), pyrolysis yields three distinct, valuable products:
- Char: A carbon-rich solid (similar to charcoal) that can be used as a soil amendment (biochar), a fuel source, or even in carbon capture and storage (CCS) to sequester CO₂.
- Bio-Oil: A dark, viscous liquid that can be refined into biofuels (e.g., biodiesel, gasoline substitutes), chemicals, or used directly as a heating fuel.
- Syngas: A combustible gas mixture that can be burned for heat and power, or further processed into hydrogen or synthetic natural gas (SNG).
- The ratio of these products can be adjusted by varying temperature, residence time (how long the feedstock stays in the reactor), and heating rate—making pyrolysis highly flexible.
3. Low Emissions & High Energy Retention
Since pyrolysis doesn’t involve burning (no oxygen = no combustion), it produces far fewer harmful emissions than combustion. There’s minimal NOₓ (formed when nitrogen and oxygen react at high temperatures) and reduced CO₂ compared to fossil fuel combustion. Additionally, the energy stored in the original feedstock is retained in the three product streams—unlike combustion, where much of the energy is lost as waste heat.
Pyrolysis vs. Combustion: The Key Differences
Combustion is the most familiar thermal process—it’s simply burning, and its differences from pyrolysis are stark. First, oxygen presence: pyrolysis occurs in a strictly oxygen-free (anaerobic) environment, while combustion requires abundant oxygen (aerobic) to sustain the burning reaction. Second, the primary reaction: pyrolysis focuses on thermal decomposition, breaking down the feedstock’s chemical bonds without oxidation, whereas combustion is an oxidation reaction where the feedstock reacts with oxygen to produce heat. The products also differ dramatically: pyrolysis yields three reusable, valuable products (char, bio-oil, syngas), while combustion primarily produces heat, along with waste byproducts like ash and harmful emissions such as CO₂, NOₓ, SOₓ, particulate matter, and even toxins like dioxins. Finally, energy outcome: pyrolysis retains the feedstock’s energy in its three product streams, allowing for later use, while combustion releases most energy as heat—much of which is wasted if not used immediately. The biggest takeaway: Combustion is about destroying feedstock to produce heat (with waste byproducts), while pyrolysis is about converting feedstock into valuable, reusable resources.
Pyrolysis vs. Gasification: The Key Differences
Gasification is often confused with pyrolysis because both use high temperatures and involve thermal decomposition, but the critical distinction lies in oxygen (or air/steam) use. Pyrolysis is strictly anaerobic, with no oxygen present, while gasification uses limited oxygen, air, or steam (a process called partial oxidation). This difference impacts their primary products: pyrolysis produces a balanced mix of three product streams (char, bio-oil, syngas), while gasification’s main focus is syngas production, with minimal char or bio-oil generated. Temperature also differs: pyrolysis operates at lower temperatures (400–800°C), while gasification requires higher temperatures (800–1200°C) to maximize syngas output. Their process goals vary too: pyrolysis is designed to produce multiple valuable products, aligning with circular economy principles, while gasification is focused on generating syngas for power, heat, or chemical synthesis. Finally, gasification is more complex than pyrolysis, as it requires tight control of oxygen or steam ratios to achieve the desired syngas quality, whereas pyrolysis needs no precise oxygen control.
Why Does This Matter? The Role of Pyrolysis in Sustainability
In a world focused on reducing waste, cutting emissions, and transitioning to renewable energy, pyrolysis stands out for its flexibility and circularity. Unlike combustion (which generates waste and high emissions) or gasification (which is focused on a single product), pyrolysis turns organic waste into valuable resources—keeping materials in the economy and reducing reliance on fossil fuels.
For example, plastic pyrolysis can convert hard-to-recycle plastics (like mixed or contaminated plastics) into bio-oil or syngas, diverting them from landfills and incinerators. Biomass pyrolysis produces biochar, which enriches soil health, sequesters carbon, and reduces the need for chemical fertilizers. These applications make pyrolysis a key player in building a more sustainable, circular future.
Final Thoughts
To sum it up: Pyrolysis is defined by its oxygen-free environment, three valuable product streams, low emissions, and feedstock versatility. It differs from combustion in that it converts (rather than destroys) feedstock, and from gasification in that it produces a balanced mix of solid, liquid, and gas products (rather than focusing solely on syngas).
As we continue to seek solutions for waste management and renewable energy, understanding the differences between these thermal processes is crucial. Pyrolysis isn’t a one-size-fits-all solution—but its unique characteristics make it a powerful tool in the sustainability toolkit.
Have you encountered pyrolysis in action? Whether it’s biochar for gardening, pyrolysis oil for heating, or plastic waste conversion, share your thoughts in the comments below!